Webinar
The Future of the COVID-19 Response: The New Administration, Vaccines, and the Health Care System
Time & Location
With a new administration and a highly successful vaccine in the making, panelists will discuss what we can expect in the next few months for health care. COVID-19 outbreaks are rapidly rising across the country and experts warn of a winter that could be the most deadly phase of the pandemic. The pandemic has created rapid and unprecedented challenges to the United States’ health care system, as well as new opportunities for innovation and collaboration. Our expert panel of speakers explored the path forward and solutions for where we are now. Speakers discussed:
- What’s next at the federal level COVID-19 response, including vaccine distribution, testing, and the economic response
- The COVID-19 response at the state level, including policy and plans for reducing transmission and for equitable vaccine distribution
- Plans for further vaccine development, distribution, and addressing vaccine hesitancy
- How health plans are planning for 2021 in terms of testing, vaccine distribution, and ensuring continuity of care for members
Kathryn Santoro (00:00:01):
Thank you so much. I'm Kathryn Santoro, Director of Programming at the National Institute for Health Care Management Foundation. On behalf of NIHCM Foundation, we want to extend our sincere thanks to the healthcare and essential workers on the front lines of the pandemic for keeping us safe and thank you to everyone for staying safe and staying home. Our goal today is to share information and guidance on the COVID-19 response in the public and private sector, including action at the federal and state level, plans for vaccine distribution and testing.
Kathryn Santoro (00:00:36):
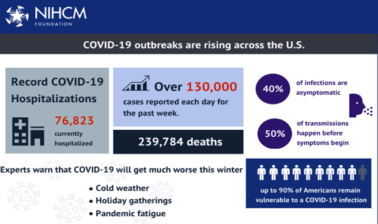
As COVID-19 has surged in recent weeks, we are seeing record highs in reported cases each day and hospitalizations. Today, there are reports that the death toll is at or close to a quarter million Americans. While we are all encouraged by the news that two vaccine candidates are showing 94.5 to 95% efficacy, many questions remain. Can we overcome vaccine hesitancy? What are the plans for vaccine distribution and addressing potential supply chain problems? Can we ramp up testing to ensure all Americans have access to regular, reliable, and free testing? To learn more about the path forward, we are pleased to have a prestigious panel of experts with us today. Before we hear from them, I want to thank NIHCM's president and CEO, Nancy Chockley, and the NIHCM team who helped to convene this event today. You can find biographical information for all of our speakers along with today's agenda and copies of slides on our website. We also invite you to live tweet during the webinar today using the #COVID-19response.
Kathryn Santoro (00:01:47):
I am now honored to introduce our first speaker, Dr. Michael Osterholm. Dr. Osterholm is the Director of the Center for Infectious Disease Research and Policy at the University of Minnesota and a member of President Elect, Joe Biden's, new coronavirus advisory board. Dr. Osterholm has been a specialist in pandemic preparation for decades. He previously served as Minnesota chief epidemiologist, and co-wrote the New York Times bestselling book, Deadliest Enemy: Our War Against Killer Germs. Earlier this week, in recognition of the grave risks that healthcare workers face during COVID-19, he announced the launch of the Frontline Families Fund to support the families of healthcare workers who have lost their lives to COVID-19. We are so grateful he is with us today to share his perspective on the state of COVID-19 and what to expect in the months ahead, Dr. Osterholm.
Michael Osterholm (00:02:46):
Well, thank you very much. It's a real honor to be with all of you and to all of you, essential workers and frontline healthcare workers, thank you, on behalf of all of us, for what you're doing. This is a difficult time in the work that you're doing and have been doing and will continue to do is critical to getting through this pandemic. Let me first of all just add a context to this discussion. In 1918, when swine flu, the H1N1 strain emerged and caused its horrible pandemic that it did back then, the average community was impacted only between 6 to 10 weeks from start to finish, and was over with. People don't often think about that as it relates to this pandemic where we're now literally into our 10th, almost 11th month of responding to this. And the challenge is that that long-term response need plays in how we think about where we're at, where we're going is really, really a remarkable, difficult step to go forward with because of just the fact that people are tired and people wonder, will this ever get over?
Michael Osterholm (00:03:56):
Well, let me just start out by adding context also and saying that today is a day of great news. We learned this morning that the Pfizer vaccine, which we'd already heard was 90% effective. Actually now is closer to 95%. I'll comment more on that in a moment, but that like it's companion vaccine, the Moderna vaccine, which the data were shared earlier this week, both not only cover the milder illnesses preventing cough, fever and chills, but actually both showed statistical protection against severe disease. This is absolutely great news. And so we have to keep thinking about that there is light at the end of this tunnel, and that is a very, very important message to share out there. It's not going to come overnight. It will still be months before we can realize many of us having access to this vaccine. But it's all the more reason why to tell people now is the time that we really have to dig in, do what we can, what we must to prevent additional transmission, to get us to the day of the vaccine.
Michael Osterholm (00:05:03):
That then gets us to where we're at today. Well, unfortunately, I believe we are at the single greatest public health crisis in the world since the 1918 pandemic. What we're seeing here, particularly in the United States and Europe is an out-of-control pandemic transmission, what we call exponential growth. Just to remind people, when you think about the numbers of cases we've seen in this country to add perspective to what's happening, when we were literally house on fire activity back in April with New York City being the focus of that ground zero, but also in areas like Chicago, Detroit, New Orleans, at that time, the highest number of cases recorded per day were 32,000. Then based on activities to try to limit transmission distancing, as some would say, flattening the curve, we got down to about 22,000 cases a day on Memorial Day.
Michael Osterholm (00:05:57):
And then pandemic fatigue starts to set in, particularly in certain areas of the country, areas that had closed down part of their economy then brought it back, and we watched the case numbers rise to almost 67,500 in that period of mid to late July. Again, particularly in states like Georgia, Florida, Texas, Arizona and California, efforts were put into place to try to limit contact, to slow down the transmission of the virus from limiting large group sizes, etc., and then we got case numbers back on this time to 32,000 cases roughly around Labor Day. In fact, at that point, you think to yourself, wow, that really is the same as it was at the height back in April. In fact, the cases bottomed out the week after Labor Day at 26,000 cases a day. Today, we're reporting out 161,934 new cases, but we've had days in the past week of over 185,000 cases. Today, we are reporting 1,707 deaths, which is now beginning to approach that of the worst of what we saw in April.
Michael Osterholm (00:07:14):
These numbers are going to continue to increase substantially. It will only be a matter of time before we hit the 200,000 cases per day mark, and go from there, unless we see major changes in the behavior of our citizens throughout the country. At this point, the cases for the next three weeks, particularly those requiring hospitalization and those who will be in ICU, and it's already in the pipeline, people who are infected today will in fact start manifesting their symptoms in five days or so from now. Five days after that, we're being hospitalized, and then potentially several weeks after that, before they die in an intensive care unit or hopefully recover. So even if we could shut off all transmission right now at this moment, we would still see this pandemic unfold really unchanged through at least the next three weeks.
Michael Osterholm (00:08:10):
The challenge we have as you know is in our healthcare systems. What are we going to be able to do? We are stretched. We are so stretched. I also realized that we are confronting what I call the trifecta of shortages. Basically, number one is workers. We know, and we surely have followed this, and as you mentioned, the Frontline Families Fund that I helped start to help cover expenses and provide support for those families who have lost loved ones who are healthcare workers to COVID-19. We know that right now, the illumining factor is not just people. And the quality of the care that people are going to get in hospitals, patients, is obviously going to be compromised just by the lack of adequate staffing, and that's number one.
Michael Osterholm (00:08:58):
Number two is PPE. I can tell you, we are going to see increasingly major shortages of PPE over the days ahead as we are consuming so much of it trying to provide care to those coming in. Also, something that happened in July here in this country was increasing cases, but what didn't happen was an increasing number of cases in Europe at that same time. And now Europe is in the same position we're in and they too are consuming these same goods, these PPE that we want. And so that's even a bigger hit on the PPE availability. The third shortage of the trifecta is that of drugs. Our group has been very actively involved working on the issue of drug shortages for several years. We've identified 40 very critical drugs for the treatment of COVID-19 patients, not the monoclonal antibodies, etc., not those, but those are just everyday needs such as antibiotics for secondary bacterial infections, Propofol, for example, in terms of trying to intubate a patient. And the 40 critical drugs we need right now as of today, 29 are in shortage status, and that number is growing rapidly, not just in terms of total drugs, but also the severity of the shortage.
Michael Osterholm (00:10:21):
Again, Europe needs those same drugs, and many of them are coming from Asia, India in particular, which has been hit hard itself with COVID and has limited production and shipment of these drugs. I can anticipate over the upcoming days that between the shortage of healthcare workers, the shortage of PPE and the shortage of drugs, the challenges will only become more acute. Now, that's hard to hear, but we have to plan for it. We have to be there. And that I think is one of the important issues we'll discuss today is what does this mean? What are the considerations we have to have? We need a national plan right now for driving down these cases. How are we going to respond? Right now we have 50 governors, many, many large city mayors, all coming up with their own ideas, their own approaches to trying to limit transmission. Will you close a bar at 10 o'clock at night or not? Do you limit sporting activities in high school? What you do with restaurants and bars if you do more than just close it, 10 o'clock at night.
Michael Osterholm (00:11:25):
I could go on with the list, but the bottom line is we don't have a comprehensive and proactive, ongoing program to understand what we're doing. We look at other countries in Europe, they surely have taken much more extreme measures to try to drive their case numbers down. The good news out of Europe is that the case numbers actually fell 10% yesterday, but they are still overall, still quite high in number. The death toll continues to increase. However, again, as you know, that severe illness and death are often leading indicators that come after the cases themselves. I just want to leave this call here and pass on the Baton with the idea that we do have the potential for a future that looks so bright with regard to this virus.
Michael Osterholm (00:12:18):
But in the meantime, we've got a lot of hard work to do, and we're asking people who are already very tired, people who are already very stressed, people who have given so much in the healthcare area. And now, unfortunately, we have to come back and ask for even more. But at this point, that is what is going to help us get to the end of this pandemic. In the meantime, however, we can all communicate with our friends, our neighbors, our families, about why the prevention efforts that we recommend are so important.
Michael Osterholm (00:12:49):
Bottom line is stop swapping air, stop swapping air, however you do that, by distancing, masking can help. The bottom line is that that's what we have to do. If you're going to go to bars or restaurants, if you're going to go to weddings and funerals, if you're going to go to church groups, high school athletic events, just know you're putting yourself, you're putting your loved ones and you're putting the healthcare workers of this country at risk. And so I hope that we all collectively can come together and help stop the transmission in this country, but still knowing even with all of our efforts, we've got some very difficult days ahead. With that, I'll stop and turn the program back over. Thank you.
Kathryn Santoro (00:13:35):
Thank you so much for your leadership and commitment to supporting the nation's critical response to COVID-19. Our next speaker, Hemi Tewarson, will discuss the COVID-19 response at the state level, including equitable vaccine distribution. Hemi is a Visiting Senior Policy Fellow at the Duke-Margolis Center for Health Policy. At the center, she's responsible for strengthening engagement and state level COVID-19 response, including such areas as new models of care and effective strategies for testing and containment. She previously served as the director of the health division of the National Governors Association - Center for Best Practices. Hemi.
Hemi Tewarson (00:14:20):
Thank you so much, Kathryn, and it's great to be with all of you today. That was a really, I think, thoughtful and sobering perspective from Dr. Osterholm. But today, I wanted to focus on his comments around vaccines, and that's really what I'm going to be talking about in terms of the state perspectives and where we've been. Before I get into sort of all those details, I'll just make one comment about state leadership on this. They really have to lead. Because there wasn't a national plan, governors were just propelled forward having to deal with things like in the early days of shortages of PPE and shortages of beds in the ICU, the ventilators, and identifying how to get their arms around testing when there wasn't enough supply. There have been a lot of challenges. And to follow up on the point of fatigue, states and their public health leaders and all of the staff that works for them have been really under a lot of pressure. It's been putting out fires since last March and it's still that way. And so recognizing that this is sort of a marathon, not a sprint, is really, I think, part of what we're all trying to figure out how we can support states.
Hemi Tewarson (00:15:36):
I did my goal lists. I'm continuing my work of really engaging state leaders on different topics, testing is one. But really today, I'm going to talk about our work on vaccine access and distribution. One of the things I wanted to talk about is how are states really getting ready for this? Dr. Osterholm mentioned really great news that the Pfizer and Moderna effectiveness numbers, and I think we're all expecting a vaccine EUA to come in December. But when that authorization comes, the vaccines are expected to be distributed within 48 hours of that event. Given the numbers that we're talking about, that is a massive undertaking, and states and localities and their partners at the health system level and the community are doing their best to really get ready, but this is an unprecedented event and it is not like just doing a flu campaign or vaccines like we've done in the past. It is a whole new way of thinking at a massive level.
Hemi Tewarson (00:16:38):
I thought I might just start to talk a little bit about who will get this vaccine, and how do we need to think about that in terms of getting ready. The National Academy of Medicine has recommended a phased approach. And using that phased approach, phase 1a and phase 1b are really going to be those high-risk health care providers, those that are on the frontlines really confronting the most risk of transmission and first responders. Phase 1b are people with high clinical risk and older adults in congregate settings, because we've seen such a high mortality rate amongst those populations.
Hemi Tewarson (00:17:14):
And then as you get to phase 2 and phase 3, which I'll talk about in a minute, you kind of broaden out the scope of getting to teachers and childcare workers and other workers in critical societal industries and with exposure risk, and people with moderate risks, etc. And then phase 3 is really where you get to the healthier population, so young adults and the children who haven't had quite the prevalence of the disease as it appeared in the older populations and people with comorbidities. And then you get to everybody else in phase 4.
Hemi Tewarson (00:17:44):
If you look at this slide here, you'll see, [inaudible 00:17:49], there it is, how there's going to be the limited doses available in the beginning, and then there'll be more, and then we will continue on. And I think what we're focusing on right now with our work with state leaders is really that first piece, how do we really make sure and help them get prepared for what is coming in this next really month of before we see an authorization from the FDA? [inaudible 00:18:17]. In terms of just the number of them, it'd be helpful just to briefly review. When we talk about phase 1a, which is not just the National Academy of Medicine, but also CDC put out these estimates as well, they are looking at, just looking at healthcare personnel and first responders could be anywhere between 17 and 20 million of the population, and you see who's covered under that with people at risk.
Hemi Tewarson (00:18:39):
I think for the states, what they don't know yet is how many vaccines are going to come to their state. So depending on how big their state is, how many of these different types of providers they have, states are having to think about sub categories within the big category, and I'll talk a little bit about that in a few minutes, about the challenges of making sure that you're ready, but you don't know exactly how many you're going to get, which is a little bit of chicken and egg determining how to prepare.
Hemi Tewarson (00:19:11):
If you look at phase 1b, which is still the first phase, but the second tier, that could be more than 100 million people with people at high clinical risk and older adults. 19 to 20 million of those folks may be at the highest risk because they have multiple comorbidities. And then you have another group, that's qualifying older adults, over 17 million, but it could go up to 53 million if you include all of above 65 years of age. Okay.
Hemi Tewarson (00:19:41):
When you get to phase 2, you can see you get to the teachers and the essential workers and moderate clinical risk, homeless folks, people in prisons, and all other older adults. The estimated population is greater than 38 million, but could be more likely to be 100 million. I just share these numbers because I think it illustrates the challenge we have in front of us as a country of getting people vaccinated at the numbers that we're really thinking about, and I think it is part of the critical solution to really ending the pandemic, but this is going to be over sort of a long period of time, not in the next month, not in the next two months, but really over the next year, really being able to get to everybody. You can see here phase 3, when you get to the young adults, children, essential workers with moderate risk, you're at 152 million.
Hemi Tewarson (00:20:32):
To get to the part about states, states were required to submit their plans for vaccine distribution and access to the CDC back in October. One of the things that we are doing here at Duke-Margolis is we're partnering with the National Governors Association to review all of the plans and really identify kind of what are the key themes, where are states starting to strategize around these different challenges, and how can we make this sort of a useful resource for when states continue to refine their plans, which they are all doing, those were just interim planning documents, they had a month to do them with not complete information, and now, they're continuing to refine them as they get more information and more understanding about what's to come. If you're interested in this topic, we will have a paper coming out in December, and happy to coordinate through NIHCM to make sure that people can get access to the paper. It will be publicly available.
Hemi Tewarson (00:21:26):
But anyway, back to this, in terms of what states are doing to actually plan, in the plan, there were different categories that they had to address, which the CDC required them to do, and one was, how do you identify and allocate vaccines to critical populations? You just saw with my previous slides, really even just focusing on the phase 1a, there could be potentially a lot of people in that phase, and understanding who they are and how you're going to get the vaccine into their arm, the last mile, which the states and localities are going to be responsible for is going to be the challenge they're going to face when the vaccine is authorized in December.
Hemi Tewarson (00:22:03):
Along with that is how are they going to do some logistical planning to meet vaccine storage and handling and administration requirements? How are they going to support vaccine provider enrollment, making sure you have enough people enrolled who can administer the vaccine, vaccine ordering and distribution and storage, which we'll talk a little bit about in a few minutes. The Pfizer vaccine, for example, requires pretty significant cold storage, and so not every facility is going to have that capacity. So figuring that out ahead of time when you perhaps don't know the exact number of vaccines that you're going to get. And when you're thinking about places like rural populations and providers who do not have that capacity, how do you handle that?
Hemi Tewarson (00:22:46):
And then engaging providers, partners, and communities and communication. This piece, I'm going to spend a little bit of time on as well. I mean, really, we have seen a lot of concern around the vaccine and whether it's going to be safe to get the vaccine, and people who perhaps were not vaccine hesitant really coming forward as vaccine hesitant. There has to be a lot of work done on really being able to effectively communicate about, here is why it's important to get the vaccine. Here is the science that we know that supports the safety and efficacy. And here's how you're going to be able to get it over time.
Hemi Tewarson (00:23:20):
I mean, I think the other part of the messaging that we're really going to have to focus on is not everybody is going to be able to get it in that first month, even within those groups that I showed you earlier. And so making sure it's clear to the public how this is all going to work is going to be critical, and it will be critical for us to get it right in the first instance as opposed to not getting messaging out until later. Things happen and things don't go perfectly. They never go perfectly, no matter how hard there is an effort to plan for them, but being able to make sure that there's an understanding of how this is all going to work is going to be critically important.
Hemi Tewarson (00:23:56):
Okay. [inaudible 00:23:58]. Okay. I just wanted to focus a little bit on some key challenges. Before I get to this identifying and allocating to the critical populations, I wanted to talk big picture about a few things that states have raised. Given the, sort of the massive nature of this vaccine effort, which you saw with the earlier numbers, funding, funding is going to be a huge issue for states. Dr. Redfield himself has estimated that we need 5.5 to 6 billion dollars to help fund this effort for state and localities across the country. They're going to need funding for things like making sure they have enough personnel to administer the vaccine and educate folks, to make sure their data systems are going to work, and to really engage in a communications campaign that's of critical importance.
Hemi Tewarson (00:24:42):
I think the other challenging piece is there's really need for additional guidance for states. We're all leading on complete information about the vaccines, and on the safety side, really understanding it's going to be safe for which populations. And so that's going to be, I think really important for getting the public trust of who should get the vaccine first, and should I be signing up and be talking to my neighbors about why it is a really good thing to get this vaccine.
Hemi Tewarson (00:25:10):
The American Committee on Immunization Practices or otherwise known as ACIP is going to be, I think a key element of this. They're really looking at the EUA that FDA issues, obviously it's authorization for the vaccine, and being able to provide guidance as to here's the recommendations on who should actually get this. A number of states are waiting for this. They really would like to see the ACIP recommendations before they launch in their states. And so I think the hope is ACIP will act very quickly after we see EUA to make sure that there's that piece of evidence that states can rely on.
Hemi Tewarson (00:25:42):
I think states also are really hoping for very quickly to understand the criteria for the initial allocations. What are the factors we're going to look at? Is it going to be total population, number of healthcare workers, number of people in nursing homes? Is it going to be any element of disease transmission or prevalence? How does that all going to fit together? So they can have a sense or a guess of how many vaccines they may actually get, which can help them then plan in terms of we're partnering with different health systems and here's what we think we can get, and here's the percent of the population we're going to be able to reach in this first push, especially as I said, my expectation of quick movement after that EUA is initially issued.
Hemi Tewarson (00:26:26):
Data requirements, I think there's a lot of questions around how do we understand more of the data requirements so we can make sure that our systems work as they should in that we can share required data with the federal government, as well as share across states. I'll talk a little bit about that too. And then of course, they want more information on communication resources and provider training. One thing with different types of vaccines, I think it's going to be fantastic because there'll be more vaccines for more people, but they have different requirements. So how do we train those individuals so they understand the different requirements of like a cold storage versus a vaccine that doesn't need that type of cold storage, two doses for a vaccine that doesn't need two doses, etc.
Hemi Tewarson (00:27:03):
I really should probably keep moving on because I'm going to run short on time, but there's also a couple of specific factors I wanted to just highlight. There are some states that have a lot of rural areas, for example, so workforce capacity and rural locations have cold storage, and how do we reach populations is going to be particularly challenging for them. There's also certain weather factors of how do you think about mass distribution sites, if you have a state that has pretty severe weather. Okay. Maybe that's not a reasonable way to think about it. And then there's also public health governance, which is whether it's centralized or decentralized, and how that can impact the work that states have to do to actually get the vaccine into the arms of people.
Hemi Tewarson (00:27:46):
Okay. So just briefly on the identifying and allocating early vaccines to critical populations. We know the initial supply is going to be limited. The framework I showed you earlier will have to be adapted to state conditions, population and infrastructure. States are going to have to develop a clear, transparent process for allocation, getting input from both the federal government and the community, and really using equity at the forefront. I think there's going to be a lot of hard decisions depending on how many vaccines a particular state gets and how large they are and how much of the population they're going to want to serve.
Hemi Tewarson (00:28:22):
A couple of examples just in terms of how states are trying to get their arms around this. Some of them have established advisory committees to try to help make sure that they're going to be able to make the right decisions very quickly. Some have already decided, look, we know we're not going to have enough for every healthcare worker at risk, so we're going to prioritize people who have two or more comorbidities. There's counties that really have just elevated [inaudible 00:28:48] transmission. We're going to try to start there first. Other states are doing surveys of providers and provider organizations, and working with their hospital associations to say, hey, can you tell us who you think are going to be the people that you think are going to be most critical to actually get the vaccine? There's different, I think, strategies across the spectrum, but there is going to be a real need to make sure the right population is identified quickly.
Hemi Tewarson (00:29:12):
Let me just spend a minute on talking about operational planning. I talked a little bit already about the cold chain storage challenges and how the Pfizer vaccine was going to require a second dose. You're going to have to make sure that people are following up and get that second dose. A couple of things I also wanted to mention here is recruiting and enrolling providers. This is going to be key, and states of course, going to leverage the providers that are involved in their vaccines for children program and their existing state Immunization Information Systems, otherwise known as IIS. But really in some states, they had to think about, okay, who is going to have that cold storage capacity? Is it going to just be the larger health system and pharmacy chains, for example, initially?
Hemi Tewarson (00:29:57):
As we think about sort of down the chain of when more and more people have availability for the vaccine, there's more vaccines available, the states also are going to think about non-traditional providers, and some states have already said, we're going to expand our scope of practice for professionals so we can make sure that EMTs and paramedics can administer vaccines. We're going to look at other types of providers who can administer these vaccines, and thinking about even down the road, when we really have to get the public trust for really being willing to get the vaccine and also access, right, making sure access is available for all the different populations, including communities at risk where there is black Americans and Latinx populations, where there is right now a fair amount of distrust of the vaccine. How do we get it to those folks? And there are different considerations. Some states are going to of course rely on mass vaccination clinics and primary care providers and pharmacies and local health departments, but they're also thinking about maybe somewhat non-traditional providers like mobile units, harm reduction sites, churches, places in the community where people feel like they can go.
Hemi Tewarson (00:31:06):
The other thing I did want to note too is all of this distribution sites initially will be closed point of distribution. They are not going to be open to the public. They're really going to have to be limited to getting it to who is actually going to fit the criteria.
Hemi Tewarson (00:31:25):
Okay. In terms of data systems, I just want to spend a minute on this because this is going to be... states are working on this and will continue to be working on this with the federal government. States all have Immunization Information Systems. The challenge is given the sort of massive amount of vaccinations that are going to have to be given to folks, some of the current systems that states are not... they're not going to be able to handle the volume and all the capability that's going to be needed for this vaccine. Some are thinking about, okay, we can use our current systems and add some additional complimentary functionality. Others are saying, okay, we're going to have to integrate third-party tools. And then there's another group of states that are saying, well, we're really going to have to supplement it with the federal technology such as the CDC Vaccination Administration Management System, otherwise known as VAMS.
Hemi Tewarson (00:32:15):
There's definitely variability in terms of how they're going to be able to effectively track the vaccine, and this is really important, being able to track the vaccine, making sure people get second doses, and then also identifying where there's any adverse events because that's going to be really important information change to established, to inform the next phase of distribution, making sure we understand who gets it and what happened to them.
Hemi Tewarson (00:32:44):
One challenge I just did want to highlight for these groups, there is a system called IZ Gateway that was established years ago at the federal level, and it supports data exchange amongst Immunization Information Systems. And so in a perfect world, as it was visualized, you would be able to put information into the system, and then there could be a lot of sharing at the federal level across states of who has gained the vaccine. How are things getting impacted? I think the challenge with this is it was established a couple of years ago, but only two states currently live on it. It just hasn't kind of been implemented in a way that other states have been able to really participate. So although a number of states, and most states, frankly, say they're planning on using IZ Gateway, there's going to be some work in order to make that a reality.
Hemi Tewarson (00:33:32):
One of the pieces also is, I think have to sign data use agreements to allow the sharing of information with the federal government and others, and there has been some legal issues around whether their state laws prohibit the sharing of identifiable data. I think there's more clarity that's needed around exactly how will the data be stored and shared, and how does that comport with what may be prohibited at state level. The last thing I want to mention, which isn't on the slide, but I wanted to be sure to highlight for you is a couple of states also in their state vaccination plans identified data dashboards for public reporting.
Hemi Tewarson (00:34:09):
I think this is really important given the concerns around getting vaccines and the need to really build public trust, having an outward dashboard that shows, here's who's getting vaccinated, here's the information of where you can get vaccinated, etc., is going to be really important. And so some states have new dashboards, and they're thinking about how to build the COVID-19 vaccinations into those dashboards. Other States are thinking about different technology on a public website, etc. I think it will be interesting to see how that all plays out, but I do think there's an interest in really making sure there's transparency for the public.
Hemi Tewarson (00:34:43):
Okay. There we go. And then really, this is the last line. I'm going to turn over to my colleagues. Let me just talk a minute about vaccine communications. I talked a little bit already about how there's been kind of diminished public confidence in the integrity of the FDA approval process. I had an old headline here, because I just wanted to remind everybody of kind of where we were before election. A lot's happened since then. But where we were before election of people really worried about getting a fast approval, I think the work done by the manufacturers and some of the good news that Dr. Osterholm shared at the top of the call about the effectiveness of the vaccine, hopefully is going to go a long way in making people feel like it's safe.
Hemi Tewarson (00:35:23):
I also think once we see the actual safety data as part of the approval or authorization by the FDA, and then the ACIP recommendations that follow it, hopefully will provide a really good basis to say, this is a safe and effective vaccine for these populations, and this is why it's going to be important to make sure people are vaccinated. I think a piece of that is really making sure that the public understands, and not just from governors or mayors, but from other folks, the people they trust, their doctor, their nurse, their neighbor, where there is a conversation across the country at different levels of, what is this vaccine and who should get it and why, and how this is going to be a critical piece to solving the pandemic that we find ourselves in that is unprecedented.
Hemi Tewarson (00:36:12):
I wanted to talk for just a minute about the disparities. We all know that healthcare disparities exist and have existed for a long time. COVID-19 just shone a spotlight on it, frankly, for black Americans who in some states had mortality rates and case rates that were like five to one initially in states like Michigan. It's really been sort of stark comparison to see that, and also with native Americans and Latinx communities. And so getting to those populations and making them feel like they can trust the vaccine, and giving them access to it in a way that works, I think is going to be a critical part of us reaching those populations and really trying to address the disparities that we know exist.
Hemi Tewarson (00:36:57):
With that, I know I should turn it over back to Kathryn, and happy to take any questions in the discussion.
Kathryn Santoro (00:37:04):
Thank you so much for sharing all this important work happening in the states and what still needs to happen to promote widespread access and acceptance. We look forward to staying in touch on your continued work. Under the leadership of President and CEO, Andrew Dreyfus, Blue Cross Blue Shield of Massachusetts has invested more than $218 million to support its members, customers, clinical partners and the community throughout the COVID-19 pandemic, including more than 100 million in premium refunds. To hear more about these efforts, we are now joined by Katherine Dallow, the Vice President and Medical Director of clinical programs and strategy at Blue Cross Blue Shield of Massachusetts. Katherine.
Katherine Dallow (00:37:54):
Thank you, Kathryn. It's a pleasure to be here, and I really appreciate the comments of my prior colleagues, and hope to give a little bit of insight as to how the insurance industry has and will continue to support efforts across the spectrum. So a little context, when the pandemic hit our state, we acted immediately to balance the urgent needs of our nearly three million members, employer customers, clinical partners and the community. We waived cost shares immediately for medically necessary COVID-19 testing and treatment, covered all in network medical and mental health visits via telehealth at no cost to members. We broadened access to mental health services, knowing that would be a critical need, including actually permanently paying for behavioral health telehealth visits at in-person rates ever since, and increasing reimbursement for child psychiatry in particular. We acted swiftly to help our most vulnerable members, creating a COVID risk index that helped us to identify members with highest risk for contracting the virus based on comorbid conditions and other factors, and those who would also be at risk of experiencing severe symptoms. We used socioeconomic status, and prioritized outreach, frankly, in COVID hotspots.
Katherine Dallow (00:39:16):
To date, our nurse case managers have reached out to approximately 37,000 at-risk members to support their care. As mentioned, to support employers, we did provide $100 million in premium relief. We extended grace periods for payment, helped provide coverage to furloughed workers, and have held many webinars for over 10,000 participants on issues ranging from mental health to racial disparities, and the basics of the biology of the disease. We supported providers by suspending administrative requirements, advancing payments and creating a new value-based payment program for independent primary care physicians to improve quality, lower costs and provide financial support during the pandemic.
Katherine Dallow (00:40:01):
And finally, we did respond to the needs of our neighbors who may or may not have been our members. Our employees worked at the Boston Hope field hospital, which received a lot of press during the worst days of the pandemic to date, and with the department of public health in Massachusetts as contact tracers. We donated tens of thousands of meals from our company kitchen, and through our foundation and company, we've made more than $10 million in direct contributions to the community and the fight against COVID.
Katherine Dallow (00:40:32):
To give you some dollars and cents, our data from March to September, just in that six-month time period, Blue Cross of Massachusetts spent over $200 million on COVID-19 specific treatment and diagnostic testing. We've spent twice that in spending on telehealth, including waving member cost share, and that wasn't necessarily COVID specific mental health. That was all-inclusive. I've also seen just in those six months, telehealth visit claims spike to 4.1 million, and we're working feverishly to figure out how to maintain telehealth access since, in a way that is sustainable.
Katherine Dallow (00:41:18):
I'd like to back up a little bit. That gives you the context of what we've done as a plan sort of in an overarching fashion. But now I want to switch to the topic that my prior presenters have been speaking on, and talk about the role of the insurer as we see it in the vaccine distribution and roll out. I really cannot stress enough the importance of insurers and federal state, and municipal public health agencies linking arms and collaborating like no time in the past that we ever have. We work together today. We've worked together on many initiatives over the years, but nothing has required this level and complexity of collaboration before in modern US health insurance history.
Katherine Dallow (00:42:03):
I believe Dr. Osterholm referenced that this goes back to the pandemic of 1918. And at that point, our scientific advances in this area in vaccine medicine were minuscule and practically non-existent. So really communication and education of the public must be consistent, factual and continual. We've heard a lot over the past several years, but especially this election season about fake news. What we really need to do is band together with the new administration and make sure the facts are out there in a consistent way, and really make sure we repeat those facts and information as it's available.
Katherine Dallow (00:42:47):
We personally at Blue Cross have broad-based social media channels that can and will be leveraged, frankly, to assist our DPH on publicizing messaging to our members, providers, and the general public. We have a brand journalism website that is available to anybody. We publish articles and facts on that in repeated fashion, whether someone's our member or not, they can access that information. We've held many conversations with our local DPH awaiting some of the guidance that was just explained. This really is not about traditional employment or coverage concerns. This is about identifying people, as was just mentioned, appropriately for prioritized and staged vaccination, and ensuring follow up, as already mentioned I think what people don't really realize until they think on it is that data from many sources will be used and should be used to make sure that those who are the most vulnerable are identified and vaccinated per federal and state prioritized guidelines.
Katherine Dallow (00:43:56):
Now, some of those data sources can be direct care providers, hospitals, long-term care facilities, retail pharmacies have certain pieces of information, community centers, homeless shelters, schools and insurers, to name a few, all provide different pieces of information that can be put together to identify who should be contacted first and prioritized for these vaccines. We personally have received inquiries already from our own clients, commercial clients who are nonmedical providers as to whether we can assist as their insurance company with organizing work-based vaccination clinics as much as we have in the past for flu vaccines for years, and have done on our own campuses. While we definitely support that idea down the road, we are very clear that that is a day two or three at best consideration, and we will assist at that time. But we as a society need to figure out first how to identify and prioritize the people who can be vaccinated day one and two.
Katherine Dallow (00:44:57):
I'm going to shift a little bit to the topic that was just brought up as well on vaccine hesitancy. With regard to COVID, the numbers vary by the polls, but to give you a little bit of history, the Gallup poll that was out today, now this poll was conducted before the news about Pfizer and Moderna found that 58% of participants said they would get a COVID-19 vaccine when one becomes available. Slight rebound in vaccine confidence, after a similar poll in September found it was more like a 50-50 split, and maybe with the Pfizer, Moderna news, that will change a little bit. If you go back to October, the share of Americans who said they're likely to get a COVID vaccine was dropping and the decline was notably more pronounced among black Americans than white individuals. And that is something we are going to have to address head on.
Katherine Dallow (00:45:55):
I do want to take a step back into the history of public health for a minute and talk about vaccine hesitancy in more general terms. We have heard the word twindemic. We've seen what's happened with some areas where there are allowances for parents to decline childhood vaccinations depending on state regulations for preventable things such as measles, mumps, rubella, tetanus, diphtheria, polio, hepatitis, you name it. I think we need to take stock and think about the vaccines we have available today and have had available for other diseases during this pandemic.
Katherine Dallow (00:46:39):
If you think about it, an estimated nine million childhood vaccine doses could be missed nationwide in 2020. We have made up ground since some of the data was collected back in March, April and May when we saw a significant decrease, but that kind of statistic really threatens community protection against other highly contagious diseases and preventable diseases like polio, pertussis, measles. And we really think that by the end of the year, we are on track to have a decrease of up to 26% in childhood vaccination doses for things unrelated to COVID, which would simply compound any COVID exposure or infection, and potentially devastate communities for whom herd immunity is not maintained because of this.
Katherine Dallow (00:47:28):
According to new Blue Cross association data, 40% of parents and legal guardians say their children missed vaccinations due to COVID-19. Again, we have made great strides in trying to get those made up, but we are not going to get to the full cohort by the end of the year. We know safe and effective vaccines help prevent two to three million deaths per year, and are paramount to protecting the safety and health of Americans in the communities where we live. We don't want to make this a double whammy by missing those vaccines that exist today. To help spread vaccination awareness, we as a Blue Cross provider, continue to partner with the medical community, local health groups and others who support timely vaccinations, and we dispel the myths about vaccines as much as we can in our public websites as well.
Katherine Dallow (00:48:20):
The last thing I want to touch on unfortunately, is a topic that is fairly ugly, but that has really been front and center since the pandemic began, and many people that aren't in the insurance industry aren't as aware, but it's related to fraud, waste and abuse. It's sad to say that with all of this also comes an inevitable surge of fraud, waste and abuse activities. As we all strive to reach the most vulnerable individuals and legitimately encourage vaccinations where and when appropriate, there will be individuals and organizations doing so illegitimately, nefariously, and to gain access to personal information and, or simply sell fake products that they've produced.
Katherine Dallow (00:49:03):
Here at Blue Cross, we routinely educate members about how to detect if an appropriate level of information is being requested by appropriate entities, whether that be by paper, web, or phone, and we redoubled those efforts early on in the pandemic with regard to schemes that were focused on testing. It was rampant. And as we enter the vaccine era for COVID, it will be especially important for us all to be vigilant in the fraud, waste and abuse space during the many months of vaccination efforts, and to share as much information as possible among us and between agencies and companies with regard to those schemes, because it's the only way we will shut them down early and with the least amount of damage. We can't have vaccine hesitancy compounded by fraud, waste and abuse schemes, have people receiving therapies that they think are appropriate but are not, and simply creating misinformation and also putting people at risk of their personal data and healthcare being devastated. I'll stop there. I know we want to stop for some questions, but I did want to mention that last part.
Kathryn Santoro (00:50:12):
Thank you so much for sharing Blue Cross Blue Shield of Massachusetts leadership and commitment to education and vaccine education. It's great to hear about all of the great work that you're doing and will continue to do. We are now excited to take some questions from our audience. I'm going to start, and I will ask all of our speakers to come off of mute. We've heard a lot today about the vaccine, and we know the timeline is pretty quick for turning around this distribution. We're also hearing a lot about potential obstacles, both in terms of the upcoming presidential transition, what that means for distribution planning, supply chain issues, some states having review boards in addition to guidance from the federal government. And would love to get our speakers' thoughts on what you're anticipating as some of our big obstacles ahead, and what you see as some potential solutions as we kind of navigate this territory for the next few months. Dr. Osterholm, do you want to start?
Michael Osterholm (00:51:29):
Yeah. Yeah. Sure. I'll be happy to. I think at this point, the critical issue for distribution really lies in the connections at the state and local level. As much as Operation Warp Speed, I think has been a tremendous success in terms of bringing these new vaccines, and we should all celebrate that, I think it has been a bit more difficult in terms of forging the national direction and leadership on states, particularly state health departments, local health departments, healthcare organizations as to how these vaccines will be distributed. The last mile is so critical. I think that I see the success of these vaccines getting into people's arms in two areas. One is what will be the ultimate collaborative efforts and how will they work at the state and local level, realizing that we've already had in many instances pre-existing relationships were very important and were already very functional. I've heard from a number of state health department leaders that this has been a huge challenge. I think the same thing is true from some local health care organizations, as well as even private companies, pharmacies and so forth.
Michael Osterholm (00:52:57):
The second thing is we just, as you've heard, we have an urgent need to help educate the public about why this vaccine, one, is critical to their health and the health of their families, loved ones, colleagues, friends, etc. And two, why with what data we have right now, we can feel the swell of confidence about the safety of the vaccine, and that despite all of the potential negative sense of Operation Warp Speed, too fast, not safety, or the fact that for some time, there were a number of us that were concerned that there may be a political finger on the scale of approval process before the election, that has been removed. I have never been more confident in the FDA's ability with the world's leading vaccine research groups, regulatory science research, that we will see a very aggressive, comprehensive and fair review of this vaccine. And when the FDA puts it out, I hope that all the states that have indicated that they're going to potentially have their own review groups, which could only complicate and hold up the efforts, that in fact they accept the FDA's review of this and move forward.
Michael Osterholm (00:54:13):
So we're going to have to do a lot to help educate the public about why and now. And so I think these are the challenges I see that if we don't address them, we're going to lose unfortunately, a large number of individuals whose lives could have been saved, whose illnesses could have been avoided had they just taken the vaccine.
Hemi Tewarson (00:54:41):
Great. I think this is [inaudible 00:54:43]. The only thing I would add... I agree with all of that. The only thing I would add is Dr. Dallow's reference to unprecedented collaboration. I think that is going to be key, and if we don't get that right within the 48 hours of when the EUA is issued, that timeline is so tight, that's where I think there'll be challenging perspective, the data flow and making sure the right people are going to be getting the vaccine at places where there's things like cold storage and ability to actually provide access to the population to meet the criteria.
Kathryn Santoro (00:55:19):
Great. Thank you. We had a lot of questions to comment around, who will get the vaccine, priority populations, etc. Can you kind of just give a high-level recommendation for the audience of where they should be looking for, for the accurate information about that, because we know as we've discussed, there are some efforts out there to kind of counter what the science says, and where should people really be looking, because I don't think we have a lot of the answers yet.
Hemi Tewarson (00:55:56):
Yeah. I'm happy to answer this again. I think everybody really is coalescing around, only this is not final, but it's going to be healthcare workers and first responders as kind of the first group, perhaps some nursing home residents. I think really looking at the American Committee on Immunization Practices, ACIP, and there, after an authorization issued by the FDA, which will have its own guidance in terms of what populations [inaudible 00:56:25] for, ACIP will issue its recommendations on this is who should get it. CDC will promote those. The states are looking for those. And I think that will be where we are going to see who within that group that is going to be larger, I anticipate, than the number of vaccines that are available in that first push will be able to receive it. I think states are already making those determinations, as I mentioned, in healthcare workers who are at risk, prioritizing those who have two or more comorbidities, who are going to be the most at risk. Just kind of trying to anticipate if they don't have enough vaccines for everybody in that category, how are they going to be able to get them?
Hemi Tewarson (00:57:06):
And then I think following from that, you'll see it kind of expand out to the different groups I talked about. But my sincerest hope is that ACIP, the CDC, the state and local governments are going to be very clear and transparent around who is going to be prioritized, and partnering, as Dr. Dallow said, with their health systems or health plans and community organizations to make sure to get the word out to everybody in terms of who is this vaccine going to actually get to first.
Katherine Dallow (00:57:34):
Yeah. Hemi, thank you for that validation. It's also important, I think when you talk about the data too, people may be scratching their heads thinking a little bit about what the health plan has to do with the data. The doctors would know what people need, but you also think about people who may not see a provider regularly, and their visits are in the emergency room or urgent care, or they only use telehealth occasionally, and there's no one source of information, but we as a plan, get those claims. We might be able to put a puzzle together around a person that an individual provider or provider group may not know, and we can refer them for contact to a public health department vaccines site. Those are the kinds of puzzle pieces that need to be put together.
Katherine Dallow (00:58:27):
The community centers can identify more of the socioeconomic factors. And then we can layer on things we know about their diagnoses, or people who see three different specialists, and the information is not coalesced, we might have that. Those are the kinds of examples of things where these data sources can be put together. And again, all those details haven't been figured out, but that first phase will be very, very clear who we need to identify. Once you get into the second and third is where it gets much more nuanced, and some of that is going to depend on how many [inaudible 00:58:58] are available and making sure that those people in the first batch got their follow-up first and are prioritized for their second vaccine.
Michael Osterholm (00:59:08):
I'm sorry to interrupt, but I have to unfortunately drop off. So thank you very much for having included me. I appreciate being part of this.
Kathryn Santoro (00:59:15):
Thank you so much, Dr. Osterholm.
Michael Osterholm (00:59:18):
Thank you.
Kathryn Santoro (00:59:20):
Thank you. Just one quick question in closing for our two other speakers, we had a lot of great questions come in and just not enough time, but in the near term, we had a few questions come in about how do we save the practice of contact tracing, especially over the next few months or up to six to eight months until we have widespread vaccination. A lot of states are overwhelmed with cases. What are the next steps for that? And also, to the extent that you can discuss sort of next steps with therapeutics and potentially increasing access to therapies in the near term.
Hemi Tewarson (01:00:00):
Yeah. Happy to do that quickly. In terms of the contract tracing, there has to also be... we didn't talk about it much today because there is widespread testing. There has to be screening and surveillance testing really ramped up, and along with that, the contact tracing to figure out when somebody tests positive, who have they been exposed to. I think that is going to be a challenge. The numbers have gotten so high in some states that they just don't have the capacity, so being able to do what they can and then just do rapid testing and self isolation along with what they can do on contact tracing is going to be key.
Hemi Tewarson (01:00:34):
On the therapeutic side, monoclonal antibodies, I mean we're spending a lot of time at Duke, there's a team here really doing a lot of work on this. It is also going to be, I think, a really key innovation and being able to get it to the right folks, and that's the therapeutic where you have to get it to the person before they get really sick. So it's not as if you're going to know someone's in the hospital, oh, now they need monoclonal antibodies. It's going to be a little bit more challenging because you have to figure out the timing on that and getting them tested and then to monoclonal antibodies in really quick order, and making sure you're getting it to the right people, I think is important, but that it could also be something that's going to be really important in this response.
Katherine Dallow (01:01:16):
... [inaudible 01:01:16] from the [inaudible 01:01:17] standpoint, we've been, both the federal and state agencies have also been working with us and others on which things make sense that are medically necessary and should be just simply provided with no cost share and other things. So that's another area where we've been working very tightly with our respective agencies.
Kathryn Santoro (01:01:40):
Great. Well, thank you all. This has been a really excellent panel today, and we appreciate your taking the time from your busy schedule. Thank you to our audience for joining us. I'd love for you to take a moment to share any feedback from this event by completing a brief survey. We'll be sharing a recording of the event, and you can also view our other COVID-19 resources on our website, including an infographic on vaccines and some research summaries from one of our grantees. So thank you all again for joining us today stay safe.
Speakers & Presentations

Michael Osterholm, PhD, MPH
Biden COVID-19 Advisory Board; University of Minnesota

Hemi Tewarson, JD, MPH
The Duke-Margolis Center for Health Policy

Katherine Dallow, MD, MPH
Blue Cross Blue Shield of Massachusetts
COVID-19 Vaccines: Planning for Equitable Distribution and Access: The Duke-Margolis Center for Health Policy, October 22, 2020
Coronavirus vaccines: We address 3 big questions about safety, distribution and adoption: Journalist's Resource, August 23, 2020
More Related Content
See More on: Coronavirus